CHIsOH at 90 C 2. 10 m solution of ionic compound sodium chloride nacl b.

Which Molecules Have Higher Or Lower Vapor Pressure Youtube
Which of the following would have the highest vapor pressure.
. 56C Vapor pressure describes the tendency of a substance to change from the liquid to the gas phase so substances with weaker intermolecular forces will have higher vapor pressures. C 10 M solution of molecular compound sucrose C12 H22 O11. B 10 M solution of ionic compound potassium chloride KCl.
The vapor above this solutionis collected and condensed. The freezing point of pure benzene is 549 C. CSH13OH at 90 C c.
Water H2O100C methanol CH3OH6496C ethanol CH3CH2OH785C diethyl ether CH3OH2-O-CH2CH3 345C ethylene glycol HO-CH2-CH2-OH198C. Answered expert verified. FeCls at 25 C d.
Which of the following substances would have the highest vapor pressure at 298 K. Vapor pressure is defined as the pressure exerted by vapors or gas on the surface of a liquid. Hereof which has the highest vapor pressure.
Using the following data determine the. CsHI3OH at 25 C b. A 10 M solution of ionic compound sodium chloride NaCl.
The lowest boiling point will have the highest vapor pressure. At 25C the vapor pressure of diethyl ether CH3CH22O is higher than the vapor pressure of its isomer n-butanol CH3CH2CH2CH2OH because A diethyl ether has a higher density than n-butanol. 10 m solution of ionic compound potassium chloride kcl c.
Which of the following liquids will have the highest vapor pressure. Which of the following aqueous solutions would have the highest vapor pressure. The freezing point of a solution made using toluene in benzene is determined to be -130 C.
Which would have the highest melting point. Hence more will be the solute particles lower will be the vapor pressure and vice-versa. What is the vapor pressure at 23C of a solution of 120 g of naphthalene C10H8 in 256 g of benzene C6H6.
C diethyl ether has a lower critical temperature than n-butanol. Which solution has the highest vapor pressure. See the answer See the answer done loading.
So in the given compounds we have methanol which has hydrogen bonding which is here. Which of the following has the highest vapor pressure. The vapor pressure of naphthalene can be neglected.
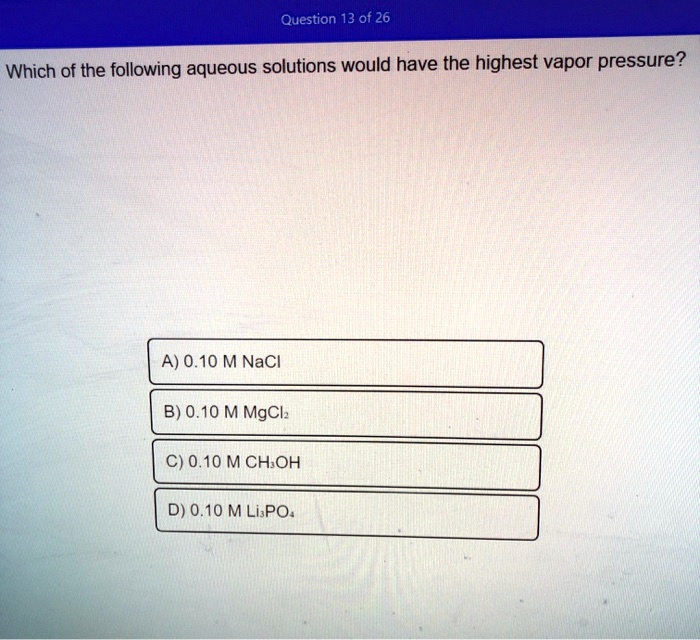
Which of the following would have the highest vapor pressure. B diethyl ether has weaker intermolecular forces than n-butanol. A 010 M NaCl B 010 M MgCl₂ C 010 M CH₃OH D 010 M Li₃PO₄.
So the boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. 10 m solution of molecular compound sucrose c12h22o11 d. For example at any given temperature methyl chloride has the highest vapor pressure of any of the liquids in the chart.
10 M solution of ionic compound sodium chloride NaCl 10 M solution of ionic comopund potassium chloride KCl 10 M solution of molecular compound sucrose C12H22O11 pure water. CO2 What is the melting point at 15 atm pressure for the. CHisOH at 25C e.
See answers 2. Water bp 100 C benzene bp 80 C chloroform bp 61 C acetone bp 56 C explanation. Vapor pressure is inversely proportional to the number of solute particles.
Which of the following liquids would have the highest vapor pressure factoring in both the impact of the substance and the temperature. So here were looking to determine which one has the highest boiling point out of the examples we have. Which of the following would have the highest vapor pressure.
The vapor pressure of pure benzene at 23C is 860 mmHg. At the normal boiling point of a liquid the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere 760 Torr 101325 kPa or 1469595 psi. 200 g of glucose C6H12O6 in 100 mL of water.
Which one of the liquids would you expect to have the highest vapor pressure at room temperature. 200 g sucrose C12H22O11 in 100 mL of water. A It dissociates to give two particles.

Solved Question 13 Of 26 Which Of The Following Aqueous Solutions Would Have The Highest Vapor Pressure A 0 10 M Nacl B 0 10 M Mgclz C 0 10 Mchoh D 0 10 M Lispo
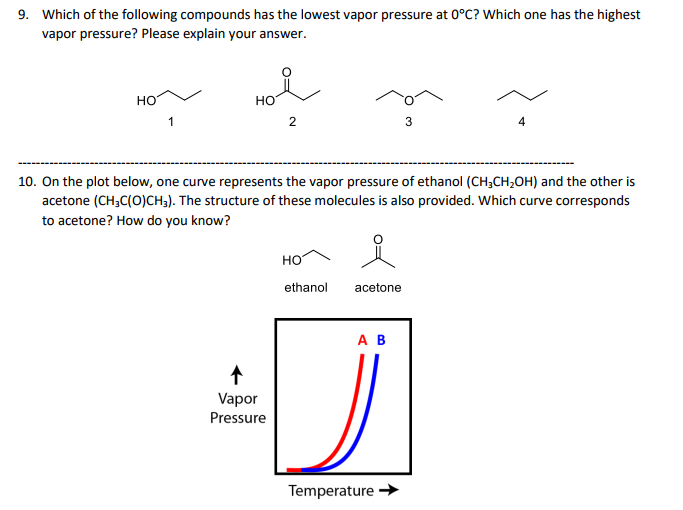
Solved Which Of The Following Compounds Has The Lowest Vapor Chegg Com

0 Comments